
Sony introduced the world’s first commercially available spectral cytometer in 2013 and paved the way for the use of this technology in the mainstream life sciences community. Over the past decade, Sony’s spectral flow cytometers have enabled researchers to use a wider variety of fluorochrome combinations than previously possible with traditional non-spectral systems. The newest addition to the Sony analyzer portfolio is the ID7000™ Spectral Cell Analyzer. This state-of-the-art system can be configured with up to 7 lasers and 186 detectors to simplify high-parameter experiments and streamline multicolor workflows.
This innovative system enables user-friendly multiparametric detection with a number of key benefits, including improved flexibility in panel design, the ability to re-use spectral references, and tools for removal of autofluorescence. The ID7000 Spectral Cell Analyzer builds on Sony’s experience with spectral analysis and simplifies many operations, even for complex experiments. With the true signal for each fluorochrome unaffected by autofluorescence or subjective adjustment of spillover, spectral analysis yields cleaner, unbiased data for every experiment.
Spectral analysis, while allowing researchers to see the full emission signal without using bandpass filters, also enables autofluorescence to be handled as a separate color. In conventional flow cytometry, cellular autofluorescence produced by pyridine (NAD/NADH), flavin (FMN, FAD), and other intracellular oxidative reactions can interfere with signals of other fluorescent markers. Other common sources of autofluorescence include cell fixation and permeabilization. Sony spectral technology can assign up to eight autofluorescent populations as independent parameters, allowing researchers to see the true fluorescent populations.
Researchers at the Pasteur Institute have published a paper that characterizes nonhematopoietic cells distinct from hematopoietic stem cells in mouse fetal liver. Using the ID7000 Spectral Cell Analyzer with the Autofluorescence (AF) Finder, they were able to identify three cell types based on their AF characteristics and demonstrated that the AF signals were actual inherent properties of these stromal cell fetal liver populations.
These researchers took advantage of the ID7000 software’s Weighted Least Squares Method (WLSM) and unmixing algorithms to subtract AF from true fluorescence signals. They detected and visualized two AF signatures, which they added as independent parameters to the original 14-fluorochrome experiment. Understanding the true AF associated with each cell population helped them design a downstream sorting strategy. RT-PCR was used to further confirm the lineage affiliation of the three cell subsets. By treating the signals detected with the Autofluorescence Finder as parameters, the researchers could identify surface markers characterizing the three cell types. They found the ID7000 Autofluorescence Finder tool useful in identifying cell subsets that were difficult to analyze by conventional methods.


At Aarhus University in Denmark, the FACS Core Facility provides cell analysis and sorting services to both university departments and external institutions using a variety of state-of-the-art instruments, including an ID7000 Spectral Cell Analyzer for high-parameter full spectrum flow cytometry. Dr. Charlotte Christie Petersen, Head of the FACS Core Facility, described the typical protocols performed in the laboratory, the factors that went into choosing the ID7000 analyzer, and the effect that it has had on everyday workflows.
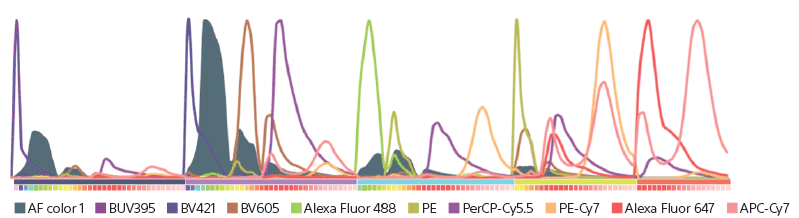
Peripheral blood contains a wealth of different cell types that can be defined using flow cytometry. Until recently, classifying these subsets has been limited because of the lack of fluorochrome combinations and available technologies to distinguish between individual fluorescent signals. This application note describes a technique that has largely overcome this issue, using the ID7000 Spectral Cell Analyzer equipped with five lasers, to classify various lymphoid, myeloid, and progenitor subsets in human peripheral blood, using 42 fluorescent markers in parallel.
Spectral flow cytometry offers more flexible panel design for multicolor experiments, because it can detect fluorochromes that might not be distinguishable with non-spectral optics, due to overlapping or highly adjacent emission spectra. This technical note describes a method using the ID7000 Spectral Cell Analyzer equipped with a 320-nm deep ultraviolet laser, highlighting the ability of this wavelength to better classify cell populations, ultimately improving the way biological samples are analyzed.
At Sony, we strive to bring you the latest innovations and deliver the highest quality flow cytometers to accelerate your research. Discover how the ID7000 Spectral Cell Analyzer incorporates our cutting-edge spectral technology to enable unrivaled flexibility in high parameter flow cytometry.


Sony attended this year’s Consumer Electronics Show to unveil its “Moving People Forward” exhibition, showcasing technologies and initiatives that connect the virtual world to live experiences. Notable exhibits included a Spatial Reality Display prototype, interactive sports experiences, and range of production activities aiming to engage and inspire creators of the future. However, the stars of the show were the mock-up and experimental simulator of Sony’s STAR SPHERE project, a remote control nano-satellite offering space-based perspectives of global issues.